


Click on sections below
Invest in ME conference
London, June 1st 2012
The conference was preceded by a 2-day ME/CFS Clinical Autoimmune Working Group meeting (click here). I was privileged to attend this meeting of 20 invited prominent researchers and clinicians. Research into ME/CFS was presented and discussed with a view to ongoing international collaboration. The main conference then followed the next day, and was held in Birdcage Walk, just behind Buckingham Palace, providing an exciting prelude to the Queen's Jubilee weekend.
Security was paramount, and the conference was opened by Dr Ian Gibson after we had had to clear the building for a fire alarm!
The keynote speaker was Professor Don Staines (Gold Coast, Australia). He presented auto-immunity as a plausible hypothesis in the aetiology of ME/CFS. He discussed the research programme being undertaken at Bond University over the
past 8 years. He began by asking the question “Is ME an autoimmune disorder?” He described autoimmunity as a reaction of the body to “self- antigens” involving B and T cells.
B cells are antibody-producing and CD20 is a potential biomarker. T cells need major histocompatibility (MHC) recognition. Innate immune responses may be triggered. (e.g. NK cells, macrophages etc). He then discussed the putative autoimmune
targets: ME/CFS is often associated with infections such as campylobacter or conditions such as Guillain Barré Syndrome. Multiple Sclerosis (MS) is associated with Epstein Barr Virus. Molecular mimicry may occur (an autoimmune response
to an organism or antigen similar to endogenous structures). In Sjogren's syndrome and myasthenia gravis (MG) acetylcholine receptors are autoimmune tagets (e.g. GPCR). Vaso-active neuropeptides(VNs) – related to glucagon, secretin, insulin
– are a super family of small peptide or protein-like molecules, and potential activators of adenylate cyclase (AC), which converts ATP to cyclic adenosine monophosphate (cAMP). VNs of particular interest and relevance to ME/CFS were discussed:
PACAP, VIP and CGRP.
They are widely distributed in the CNS. Data does support the notion of VN dysfunction in ME/CFS and symptoms do match the putative targets. VPAC2R receptors are dysregulated. There are links to FOXP3 and cAMP metabolism, and the state of receptors is crucial for transmission. cAMP is a very important neurotransmitter involved in the transmission of ATP to AMP. cAMP is a secondary messenger and has a key role in cell metabolism. It acts through CREB and ICER proteins and these have a role in CNS neuroplasticity, involving cognition, memory etc. Further discussion then focused on blood-brain-barrier (BBB) and blood-spinal-barrier (BSB) function. The BBB keeps unwanted substances out of the CNS with pericytes involved. Vasodilation controls the entry of immune cells. Interleukin-1β and TNFα are toxic to BBB and BSB and are antagonized by cAMP. GPCRs may be inhibitory or stimulatory. Autoimmunity can lead to loss of organ function. PACAP and VIP are regulatory co-transmitters. Skeletal muscle and autonomic transmission maybe implicated. They also act on the acetylcholine system. Various other functions may include: regulation of cardiac firing; an anti-apoptosis role in neuronal cells; insulin control; hypoxia regulation; glutamate metabolism Recent developments in purinergic signaling also contribute to understanding of pathomechanisms involving VNs in relation to ME/CFS. ATP signals cellular stress and negatively regulates AC.
ATP may also have extracellular effects. There are also roles for VNs in Tregs (regulatory T cells) and NK cells. Tregs have an important contributory role.
Another question asked was: Are there abnormalities in mRNA and microRNA?
Discussion then followed as to whether symptoms of ME/CFS relate to neurotransmitter function, Purinergic signalling is involved in neuropathic pain. VN function is involved in neurohormonal function, immunoregulation, and cardiotropic regulation. Pathomechanisms indicate derangements in the VN function. Abnormalities have been shown on MRI, showing disorders of micro-circulation, which are associated with onset of illness, loss of CNS function and illness severity.
In conclusion, Prof Staines feels that a novel auto-immune mechanism may be involved implicating vaso-active neuropeptides. This hopefully will eventually lead to treatments.
Dr Sonya Marshall-Gradisnik (Gold Coast, Australia) presented her work on immunological dysfunction as possible biomarkers for ME/CFS. She pointed out initially that the pathomechanism is unknown, there is no diagnostic test but there is evidence of immunological dysfunction. NK cell function is down, Treg function is implicated in ME/CFS, and research into B cells suggests an auto-immune disorder.
There is significant reduction in NK cell function coupled with significant reduction in intracellular perforin, which is responsible for NK cell lysis.
Potential biomarkers include: NK phenotypes, KIR expression, decreased CD8 cell lysis function, decreased gene expression of lytic proteins of NK cells, decreased microRNA.
She then described diagrammatically the function of the NK cells.
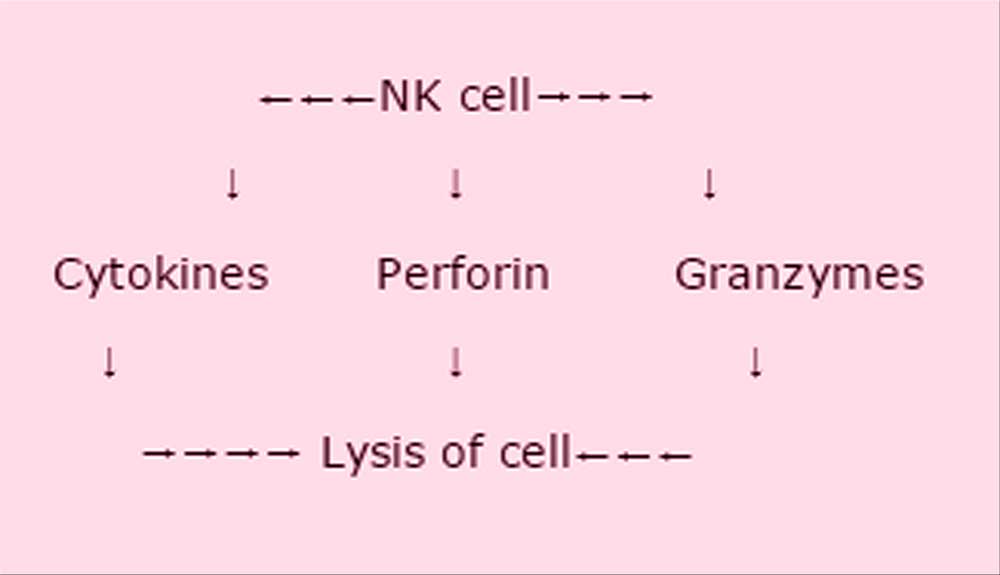
There is significantly reduced NK cell function in ME/CFS, which is consistent over time. However the function is similarly decreased in the moderately affected group, so severity is not necessarily significant. There are 2 types of NK phenotypes: dim and bright. The dim seem unaffected in CFS, the bright very much decreased. This is consistent over time in this illness. NK cells are regulated by Tregs. One KIR receptor is associated with NK cell lysis reduction. mRNA gene expression relates to GZMA lytic protein which is significantly decreased in ME/CFS – this is a potential biomarker.
MicroRNA – there is a very small non-protein coding RNA. The mechanism of post-transcriptional gene expression is based on complimentary microRNA. Differential microRNA expression is found in NK and CD8 cells. 16 microRNA studies have been performed and significant difference was found in 9 in ME/CFS. MicroRNA regulates Treg cell function. Treg cells have been measured. FoxP3 is part of Treg function and is elevated over time in ME/CFS, as is CD39 expression. VPACR2 expression in lymphocytes is also elevated in ME/CFS.
In Norway, Mella and Fluge are investigating anti-CD20 intervention using the drug Rituximab.
Results suggest that ME/CFS may be an autoimmune condition.
NK cytotoxic activity, NK phenotypes and NK proteins remain as the most consistent immunological markers. Emerging areas include: CD8 lysis, microRNA and Treg subsets. These may provide further promise for biomarker development.
Professor Hugh Perry (Southampton, UK) spoke on neuro-inflammation in chronic disease. He described how the symptoms of systemic inflammation affect behaviour. This is not maladaptive, but of survival value. This is an organized strategy and is what occurs in the wild, leading to homeostasis. Examples of effects on behaviour were given such as fever, leading to lethargy, leading to rest. The inflammatory response “speaks” to the brain. The key players are the macrophages in the brain known as microglia. The microglia are down-regulated by various molecules expressed (inhibitory receptors). In the diseased brain microglia proliferate and then are activated. Perivascular macrophages communicate with the microglia, which in turn affect the neurons.
These inflammatory changes are seen in Alzheimer's disease. Systemic infection in elderly sufferers may be acute or chronic. Worsening symptoms are associated with increased microglia and cytokines in the brain. A longitudinal study was reported in 300 Alzheimer's patients with 6 months follow up of infective episodes. The more infections reported the greater the decline in cognition.
Circulating cytokines lead to increased levels of sickness behaviour. i.e. the microglia have been primed by infection and the symptoms of underlying brain disease worsen. It is possible microglia are primed by other events. Priming of microglia can occur as a result of prior infection weeks or months beforehand. It is likely that a past history of infection can increase responses in the brain.
He described experiments where rats were infected with S.typhi systemically. There was increased cytokine activity and bacteria were cleared in 1-2 weeks. However, the pro-inflammatory cytokines continued to have effects on the brain with switching on of microglia. All the vascularity of the brain can be affected and effects persevere for months. The environment can have priming effects on the microglia. For example animals who were housed in a “dirty” environment had greater priming, with larger inflammatory response.
He said that ME/CFS seems like an exaggerated form of systemic inflammation with a distortion of homeostasis. Immune to brain communication may have become maladaptive. There may have been a primed innate response in the CNS, which may be the result of genetic predisposition/infection/inflammation/neurodegeneration.
Professor Maria Fitzgerald (London, UK) discussed pain in ME/CFS. She initially gave an overview of chronic pain, and described it as a CNS problem. Pain has a purpose and this may involve: warning, defence, escape, learning, protection, rest, healing etc. Rest is part of the healing process. Pain can be maladaptive:
- It may be poorly related to the actual “injury” e.g. migraine, fibromyalgia.
- It maybe too late to be a warning e.g. cancer
- It maybe neuropathic e.g. post-surgical, post-infection
- It causes suffering, depression, anxiety, lack of mobility etc.
- It leads to a huge economic burden.
She went on to describe the physiology and pathology of pain. It is nociceptive. Persistent pain may be inflammatory or neuropathic. Inflammatory pain is usually tender, aching and accompanied by stiffness. Neuropathic pain is usually stabbing, burning or shock-like. The nature of pain is complex:
Sensory component
Motor/autonomic component
Affective component
Can alter brain function (e.g. anxiety)
Affects attention
Individual differences
Culture, gender and age all have effects
There are multiple sites of pain processing. Pain information is modified as it travels to the brain. It can be unpredictable at every level. The nociceptors can be primed (e.g. by an inflammatory agent) leading to hyper-excitability.
In CFS/ME there is altered CNS processing. Fibromyalgia (FM) patients perceive greater intensity and greater temporal summation. Sensations increase in magnitude, and the CNS winds up more and more. There is alteration of endogenous pain control. FM patients lack diffuse noxious inhibitory control. Normally there is ability to inhibit pain (endogenous opioids). There is altered cortical pain processing in CFS/ME. Numerous areas of the cortex are involved. There is activation of the limbic system (anterior insular-based ganglia and cingulated cortex). The CNS is acting very differently.
Some people may have a genetic predisposition to feel more pain. Single Nucleotide Polymorphisms (SNPs) suggest genes related to pain sensitivity. Plans to incorporate genotyping are needed. Early life experiences may alter pain sensitivity in adult life – e.g. premature babies who have had a lot of pain exposure, may develop an altered pain threshold. “Injury “ in the first 10 days of life may promote effects that last into adulthood, leading to vulnerability, particularly if the same injury occurs, and the effects can be increasingly severe. It is not necessary to have an infection to prime the microglia, but a “wound” can lead to microglial activation.
The conclusion was that genetic determinants and early life experiences lead to nociceptor sensitisation, which is a potential cause of pain in CFS/ME.
Dr Mario Delgado (Granada, Spain) discussed his work with vaso-active neuropeptides. He said there are potential therapeutic opportunities. There is a need to establish a homeostatic balance. Cytokines are involved in the immune balance. The brain and immune system speak the same biochemical language. Vasoactive intestinal peptide (VIP) is found throughout the body and is produced in the CNS, the periphery and non-neural cells. It has a plethora of functions. It is involved in the pathway which activates adenylate cyclase (AC) which in turn impacts on ATP and cAMP metabolism. AC and cAMP signals are critical for neuronal survival and other brain function. VIP receptors are expressed in immune cells and have immunomodulatory activity.
He described therapeutic effects on experimental inflammation and auto-immunity, and finds there is helpful potential in a number of diseases. VIP protects from the lethality of endotoxins. It is neuroprotective in neuro-inflammation caused by brain trauma, and is therapeutic in collagen-induced arthritis. It also has potential for treating experimental autoimmune encephalomyelitis (EAE) (animal model of MS). In this condition it inhibits the inflammatory response and the Th1 response. It also induces the emergence of CD4 CD25 Tregs during EAE. Neuropeptides induce the generation of different types of Treg cells. VIP has been shown to be an important neuropeptide in immune tolerance.
He then asked several questions:
a) Does a healthy VIP system make us more healthy?
b) Is there a role in ME/CFS? – as many symptoms relate to the effects of VIP.
c) Is VIP ready for the clinical situation? - need to consider: side effects, stability, non-oral administration
d) What are the effects on immunosuppression?
Aviptadil (an analogue of VIP) is marketed for some conditions such as erectile dysfunction and the inflammation associated with sarcoid. VIP has also had some success in Guillain Barre Syndrome and ciguatera poisoning. A clinical trial is in progress looking at VIP by inhalation.
Professor James Baraniuk (Washington DC, USA) discussed potential mechanisms of ME/CFS symptoms. He had designed a simple questionnaire using the Fukuda criteria and looked at severity scores. He had produced a 3D diagram and had divided his findings into 4 groups. The ME/CFS symptoms could be clustered, and matched the cerebral spinal fluid proteomics.
He then went on to discuss the symptoms seen in ME/CFS in general.
a) Headaches He found the prevalence of migraine headaches to be 75-80%. 2/3 of these had no aura, and 1/3 had aura. There was an accompanying range of symptoms. There was improvement with triptans. 67% of those with migraine were found to also have fibromyalgia. There was also a high prevalence of these symptoms in Gulf War Illness. Migraine can cause abnormal cortical depolarization and abnormal pain processing, and accompanying central desensitization. He hypothesised that migraine is initiated in the brainstem.
b) Pain and tenderness There was increased sensitivity to deep pressure in FM. There was also increased sensitivity of proprioceptive and stretch receptors on nerves innervating the joint capsules, tendons etc. There maybe loss of the anti-nociceptive system, and loss of norepinephrine release, which normally regulates responses, and initiates autonomic responses. It is as if “the light is on but no-one is home”, and the brain is in a default mode pathway. He looked at pain dolorimetry – pressure-induced thresholds, and did frequency analyses. Central sensitization starts in the periphery – there maybe peripheral sensitisation such as by hay fever. Spinal sensitization leads to hyperalgesia and allodynia. Microglia are the key players, and microgliosis occurs in pain. There is potential for microglial-neural biomarkers and useful therapeutics. Potential treatments discussed were: Reservatrol (in red wine), antipsychotics, tricyclic antidepressants, anti-oxidants, endocannabinoids (medical marijuana).
c) Brain fog – He discussed the findings in the grey matter, in particular the midbrain reticular substance and periaqueductal grey matter. Changes indicated that the body's alarm clock is damaged. There was decrease in the white matter depending on the duration of fatigue, and it was found to shrink by about 1% per year.
d) Sleep It is likely the brainstem is involved
e) Effects of exercise With exercise, the brain fMRI shows improvement in healthy people. He then described 2 types of people with CFS: “Increasers” who on day 2 of exercise have to work very hard to improve their mental score. And “decreasers”, whose fMRI shows a lot of activation on day1, but no task activation ability on day 2. They are in default mode only.
The conclusions in this presentation were: That fatigue is the result of all symptoms, that headache is a result of myalgia and that general symptoms are due to a fundamental effect in the brainstem.
Professor Olav Mella and Dr Øystein Fluge (Bergen, Norway) did a joint presentation looking at B cell lymphocyte depletion in ME/CFS. They reiterated how 3 original lymphoma patients who coincidentally had ME/CFS had improved remarkably following treatment for their lymphoma with the B cell targeting drug Rituximab. In these patients all symptoms relating to their ME/CFS diminished with the treatment. B cells may have something to do with central mechanisms. The responses however were delayed for up to 6-12 weeks, despite B cells being cleared in about 2 weeks.
A further larger control study was undertaken. Patients in the study group were infused with 2 infusions of Rituximab 2 weeks apart, and the controls were similarly infused, but with normal saline. These patients were then followed every 2 weeks for 12 months. In all patients there was a strong family history of autoimmune disease. Side effects were infrequent – in 2 treated patients, their psoriasis worsened. 2 patients felt “unease” and some insomnia. Several patients had normalization of a previously abnormal menstrual cycle. There was a positive response in 67% of patients. There was a 13% response in the placebo group. Again there was delay in response and after the effects wore off the response declined but was re-instated at the 2nd infusion. Some patients however continued to improve but there was not a consistent pattern. BAFF (B cell activating factor) was lower in CFS patients than controls after treatment. BAFF is increased in auto-immune disease. BAFF was again increased at 3-6 and 8 month follow ups. This is the expected normal feedback mechanism. Psychological symptoms did not change indicating this was therefore not a psychological illness.
There are ongoing studies.
1) 26 patients to be observed for 15 months. This is a subjective study without controls.
And 2) A severe group of 6 patients. Plasma exchange may be given before treatment with Rituximab. Patients will be given 2 infusions 2 weeks apart, with maintenance therapy at 3-6-10 and 15 months. Follow up will be for 3 years. Non responders will be given etanercept weekly subcutaneously for a year. They are also looking at a study on genetic predisposition in 3 families. As yet there is no clear candidate as a plausible target for an autoimmune process. Over the past 3 years they have looked for a specific autoantibody, but have found none so far. Something else may be triggering a pro-inflammatory state.
They reiterated that patients should not be treated with Rituximab outside clinical trials.
The next presentation was by Professor Indre Ljungar (Stockholm, Sweden). Her focus was on a one year experience of a standardized team-based assessment of suspected ME/CFS. The aim of this ME/CFS project was to improve diagnosis, transfer clinical knowledge to primary care, to establish rehabilitation methods and to conduct research. The project was established in 2010 and used a team based approach such as used in other diseases. A multidisciplinary team was involved consisting of a doctor, nurse, psychologist, physiotherapist, occupational therapist and social worker. Diagnosis was based on the Fukuda and Canadian 2003 case definitions. Every patient had a full medical workup, and a wide range of tests were used. Included were blood and urine analysis, polysomnography, 3T brain MRI. Also included were 2-day activity scores, sleep questionnaire, WAIS, SF36 etc.
In the past year 101 patient visits were referred for evaluation, and ME/CFS was suspected in 55%. Of these 33 fulfilled the criteria for ME/CFS. Of the others, diagnoses included psychiatric illness, sleep disorders, neuropsychiatric disorders, other physical illnesses, fibromyalgia, idiopathic fatigue etc. The importance of multi-disciplinary team assessment, coupled with thorough medical investigation was stressed, as symptoms are very complex and there is considerable overlap with other disorders. This approach helps to create a homogenous group for potential research. Subjective symptoms can be tested objectively and reliable diagnosis helps in recommending treatment.
Dr Daniel Peterson (Incline Village, Nevada, USA) gave a clinical research update. 6000 articles have now been published on ME/CFS. The greatest challenge is the disease itself, and there is a lack of biomarkers. In clinical research, IT and translational medicine are becoming more widely used. Translational research leads to multidisciplinary collaboration, accountability and standards, shared data and data integration and common goals drive the advancement of applied science. The benefits are: larger samples, decreased cost, more controls, increased value of results, decreased time lines, and the whole approach is more manageable for clinicians and researchers, many of whom may only work part-time. The disadvantages are that technology is forever playing catch-up and there is almost too much information generated.
He went on to describe several new projects:
a) XMRV/MLV – funded by NIH at 5 sites, using a centralized lab. Results will be published at end of June 2012. Samples have been established, so may be open to further research.
b) Chronic Fatigue Initiative pathogen discovery project to establish a biobank. There are 40 patients and 40 healthy controls at 5 sites. Samples of blood, saliva, urine, faeces and tears will be taken. This will help to establish subsets, which is particularly important for pathogen discovery. A centralized biobank will be established and samples kept at minus 80̊C.
c) Collaborative research using cerebro-spinal fluid. The goal is to look for aberrations, pathogens and markers. 30 cases are being investigated, evaluating cytokines and microRNA in the spinal fluid
d) Simmaron/Bond – molecular and cellular investigation to identify NK function.
e) CFIDS funded research projects – have established the Research Institute without Walls for translational research, and are funding 5 studies: 1) Brain imaging looking at blood markers after exertion. 2) Cognitive improvement looking at treatment of unrefreshing sleep and effectiveness of drugs 3) Epigenetic markers –(heritable changes in gene expression) 4) Brain fog, orthostatic challenges and therapeutic approaches. 5) Autonomic dysfunction looking at neuromuscular strain/central sensitization.
f) CASA (collection, aggregation, storage and analysis) – a collaborative effort between the NIH, CDC and clinicians and researchers comparing studies of ME/CFS. Using many different questionnaires is not helpful, and a consensus document is needed using standardized questionnaires. Results are otherwise divergent and often not useful. The goals therefore are to establish research standards and determine appropriate tools.
g) The Open Medicine Institute headed by Dr Andreas Kojelnik.
Dr Andreas Kojelnik (California, USA) is Medical Director of the Open Medicine Institute (OMI) – a community-based research clinic focused on chronic infectious diseases, neuro-immune disease and immunology. He feels there is much optimism now in that treatments are beginning to emerge, mainstream science is getting interested and technologies are advancing our understanding. Disease acceptance in 2012 is far better than in 1992, and he pointed out that many other diseases have been through a similar history, with understanding evolving slowly. We are in a different era now, with genetic profiling becoming useful, and accurate in diagnosis and treatment. In ME/CFS there is a syndromic research conundrum. There is a lot of overlap, definitions are not mature, there is scarcity of biomarkers, treatments are not standardized and outcome data is limited.
He described how the OMI works by creating a network between biotechnology, informantics, social networking and biosampling all linking into clinical medicine and research. There is need for convergence of these disciplines. A large network gains: longitudinal controls, plenty of samples, a lot of pilot treatments, control over protocols and standards, control over lab measurements and standards and options to use the best of the best.
He went on to describe research progress in the past year:
a) Positive studies with Rituximab
b) XMRV studies “debunked”
c) CDC funding clinical networks
d) Ampligen study published
e) Other molecular studies showing progress: e.g. spinal fluid in ME/CFS vs Lyme disease, blood findings in depression vs ME/CFS/FM
He then outlined the work being undertaken at the OMI: Diagnostic studies
a) Viral flora –. sequencing and quantitation
b) Antibody/antigen array
c) Cytokine arrays
d) Deep sequencing
Treatment studies:
a) Valganciclovir and other antivirals (NB long delays in response)
b) Anti-inflammatories
c) Rituximab
d) Rifaxamin (antibiotic) etc Aetiology studies a) Immunological studies b) Infections: bacterial, viral c) Metabolic derangements (VO2 max, mitochondria etc)
d) Environmental factors (heavy metals, diet etc)
Today, much collaboration is occurring internationally between academia, industry and government. The OMI is very involved and now has a biobank of 10,000 patients.
This conference was organized by Invest in ME, and I must thank them for producing a wonderful event. Thanks must also go to the Alison Hunter Memorial Foundation and ANZMES for enabling me to attend.
Conference Presentations from IIMEC7
IIMEC7 Pre-Conference Dinner Speech

The IIMEC7 pre-conference dinner was arranged at short notice and a
wonderful presentation was given by Norwegian journalist
Jorgen Jelstad.
His speech "Words Matter" is available on the video from the conference.
BRMEC2 - International Research Colloquium, London 2012

To raise awareness of ME, and promote collaboration, innovation and foundations
for a clearer strategy of biomedical research into ME, Invest in ME has
joined with the Alison Hunter Memorial Foundation of Australia -
in cooperation with Bond University and University of East Anglia -
to establish a Clinical Autoimmunity Working Group which met in London
on 30-31st May 2012.
Kathleen McCall
Chairman Invest in ME
There is an urgent need for a coordinated strategy of biomedical research into myalgic encephalomyelitis (ME).
Good quality collaborative research efforts lead to understanding of the disease and better patient care and education of health care professionals.
The approach to treating ME must reflect the latest biomedical research evidence and ME needs to be accepted as a mainstream disease requiring major attention
from the medical profession and research institutions.
Patients need access to knowledgeable ME consultants who can make correct diagnoses using proper guidelines and need to understand the disease in its all phases.
Invest in ME is a UK charity established in 2006 by ME patients and parents of children with ME.
The charity was set up with the objectives of making a change in how ME is perceived and treated in the media, by health departments and by healthcare professionals.
Our efforts are focused on setting up a UK Centre of Excellence which will provide proper examinations and diagnosis for ME patients and initiate a coordinated
strategy of biomedical research into ME in order to find treatment(s) and cure(s).
Christine Hunter AM
Alison Hunter Memorial Foundation
People with ME face enormous obstacles to access health care. Among the impediments over past decades has been research which has shifted emphasis to fatigue and fatigue states with scant regard for the myriad yet distinguishing neurological, autonomic, and gastrointestinal features of ME. Semantics and biased attributions continue to deny the severely ill, both child and adult, the right to care which addresses their acute and chronic medical needs without fear. The Alison Hunter Memorial Foundation was established in 1998 through the initiative of the Public Interest Advocacy Centre, Sydney. The Foundation has a primary interest in the medical, legal and social needs of people with ME and the clinical documentation of severity. The Foundation supports biomedical research.
INTERNATIONAL SCIENTISTS EXPLORE AUTOIMMUNITY IN MYALGIC ENCEPHALOMYELITIS/CHRONIC FATIGUE SYNDROME
Wednesday 30th Thursday 31 May 2012, LONDON, UNITED KINGDOM
Medical and scientific experts from around the world convened in London on 30 and 31 May to discuss recent scientific developments in understanding myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS).
Co-Chair of the clinical autoimmunity working group for ME/CFS, public health physician Dr Don Staines stated ‘The recent discovery from researchers in Norway that an anti- CD20 B cell- depleting drug had a marked benefit in the treatment of ME/CFS has sent a clear message to scientists and medical practitioners around the world that this disease may have an autoimmune origin'.
While the clinicians who made the discovery, Dr Oystein Fluge and Dr Olav Mella and co-workers remain guarded in drawing unwarranted conclusions from the study published in PLoS late last year, further studies are now being planned in the hope of extending the study to a number of clinical sites and to increase the number of patients in the studies.
Dr Staines said �The findings of Drs Fluge and Mella and their co-workers are consistent with theories previously published that ME/CFS may be an autoimmune disease. Despite compelling evidence that this disease is linked epidemiologically to infection and the disorder possibly being a post-infection disturbance of the immune system, little funding has gone into studies of autoimmunity. This is clearly a multi-system illness which has been badly managed in terms of the research agenda.'
Experts who will attend the meeting include Professor Noel Rose, Director of Autoimmune Disease Research at Johns Hopkins Hospital (USA), Professor Stephen Miller (USA), Dr Mario Delgado (Spain) and Professor Hugh Perry, the chairman of the UK Medical Research Council Neurosciences and Mental Health Board. Immunological discoveries which may serve to act as biomarkers for ME/CFS will be presented by Dr Sonya Marshall-Gradisnik, Bond University, Australia.
Alison Hunter Memorial Foundation chunter@ahmf.org +61 2 99586285 Invest in ME info@investinme.org 07759 349743
WELCOME ADDRESS: DAME BRIDGET OGILVIE, AC DBE FRS
CONVENORS
BOND UNIVERSITY, Population Health and Neuroimmunology Research Unit, Faculty of Health Sciences and Medicine, Gold Coast Australia
UNIVERSITY OF EAST ANGLIA, Biomedical Research Centre, Faculty of Science, Norwich, United Kingdom
GRIFFITH UNIVERSITY, School of Medical Science, Griffith Health Institute, Gold Coast, Australia
ALISON HUNTER MEMORIAL FOUNDATION, AUSTRALIA
INVEST IN ME, UNITED KINGDOM
PARTICIPANTS
- Dr Amolak Bansal MD
- Dr. James N Baraniuk MD
- Dr Monica Carson PhD
- Professor Simon Carding PhD
- Dr Abhijit Chaudhuri MD PhD
- Dr Mario Delgado PhD
- Dr Oystein Fluge MD PhD
- Dr Ian Gibson PhD
- Dr Konstance Knox PhD
- Dr Andreas Kogelnik MD PhD
- Dr Richard Kwiatek MBBS FRACP
- Professor Stephen D. Miller PhD
- Dr Sonya Marshall-Gradisnik PhD
- Professor Olav Mella MD PhD
- Dame Bridget Ogilvie AC, DBE, FRS
- Professor Hugh Perry PhD
- Dr Daniel Peterson MD
- Professor Noel Rose MD PhD
- Dr Katherine Rowe MD MBBS FRACP MPH DipEd
- Dr Rosamund Vallings MD
- Professor Tom Wileman PhD
CFS Patient Advocate http://cfspatientadvocate.blogspot.co.uk/2012/06/investinme-roundtable-discussion-may-30.html
Our Sponsors for IIMEC7
Again the Irish ME Trust continued to sponsor a speaker for our international conference and we would like to thank them for their wonderful support.
IIMEC7 Pre-Conference Dinner Presentation
Jørgen JelstadNorway
New Directions in ME/CFS Research
Professor Don StainesThe National Centre for Neuroimmunology and Emerging Diseases (NCNED), Griffiths University, Australia
Professor Staines has been a public health physician at Gold Coast Population Health Unit.
He has worked in health services management and public health practice in Australia and overseas.
His interests include collaborative health initiatives with other countries as well as cross-disciplinary initiatives within health. Communicable diseases as well as post infectious fatigue syndromes are his main research interests.
A keen supporter of the Griffith University Medical School, he enjoys teaching and other opportunities to promote awareness of public health in the medical curriculum. He is now Co-Director at The National Centre for Neuroimmunology and Emerging Diseases (NCNED), Griffiths University in Australia
Transient receptor potential ion channels in the aetiology and pathomechanism of CFS/ME
Professor Sonya Marshall-GradisnikThe National Centre for Neuroimmunology and Emerging Diseases (NCNED), Griffiths University, Australia
Professor Sonya Marshall-Gradisnik NCNED, Australia
Co-director of the National Centre for Neuroimmunology and Emerging Diseases (NCNED) at Australia’s Griffith University. Professor Sonya Marshall-Gradisnik is one of Australia's foremost researchers in the area of neuroimmunology and has been instrumental in establishing the Public Health and Neuroimmunology Unit (PHANU) at Bond University. Much of her work relates specifically to autoimmunity in Chronic Fatigue Syndrome sufferers and she is regularly asked to speak to community groups on behalf of Queensland Health and NSW Health. Her research in the area of exercise immunology has also contributed to the body of knowledge relating to the effect of doping in sport and she serves as Sports Medicine Australia's national spokesperson in this area. The vital research conducted by Professor Marshall has attracted more than $1 million in grant funding and she has produced 21 peer-reviewed papers, five book chapters and one provisional patent. In 2008 Dr Marshall was joint leader of the Bond University team responsible for developing the the BioSMART program. The team was awarded a prestigious Australian Teaching and Learning Council Award (formerly known as the Carrick Award) for Outstanding Contribution to Student Learning and for the quality of student learning over a sustained period of time.
Professor Hugh Perry
Southampton University, UKProfessor Hugh Perry
Professor Perry and his team study Inflammation in the CNS and its contribution to Neurological Disease. The results of this research may help in the development of therapies to treat acute and chronic neurodegenerative conditions, which at present are largely untreated.
Inflammation biology in the brain is a complex subject and requires expertise in many different areas. The CNS Inflammation Group have collaborations with academic laboratories across the University of Southampton, the UK, as well as with laboratories across Europe.
Professor Maria Fitzgerald
Professor Maria FitzgeraldUCL, UK
Professor Maria Fitzgerald
Professor of Developmental Neurobiology Dept Anatomy & Developmental Biology, University College London. Maria Fitzgerald graduated in Physiological Sciences at Oxford University and studied for a PhD in Physiology at UCL. She was awarded a postdoctoral MRC training fellowship to work with Professor Patrick Wall in the Cerebral Functions Group at UCL and remained in that group as a postdoctoral fellow until starting her own research group in the Anatomy & Developmental Biology Dept at UCL. She became a Professor of Developmental Neurobiology in 1995 and was elected as a Fellow of the Academy of Medical Sciences in 2000. Maria is Scientific Director of the Paediatric Pain Research Centre at UCL www.pprg.ucl.ac.uk, and is a member of a number of research boards including the Medical Research Council Neurosciences and Mental Health Board, the Scientific Board of the Migraine Trust and the French National Research Agency (ANR). She is an Editorial Board member of ‘Pain’ and of ‘Pain Research and Clinical Management. Maria has published over 130 research papers and reviews in the area of pain neurobiology (taken from UCL site http://www.ucl.ac.uk/npp/research/mfi).
Transient receptor potential ion channels in the aetiology and pathomechanism of CFS/ME
Dr Mario DelgadoInstitute of Parasitology and Biomedicine, CSIC, Granada, Spain
Dr Mario Delgado, Institute of Parasitology and Biomedicine, CSIC, Granada, Spain
Mario Delgado, PhD, received his PhD in biological sciences in 1996 from the Complutense University Madrid, where he obtained a position as associate professor. In 2003, he moved to the Institute of Parasitology and Biomedicine in Granada, Spain, where he is full professor and director of the institute. As a neuroimmunologist, he is focused on understanding the bidirectional communication between immune and neuroendocrine systems. A primary objective of his research is to identify endogenous anti-inflammatory factors, mainly neuropeptides and hormones, with the aim of designing new therapeutic strategies for immune and neurodegenerative disorders. Moreover, he discovered the immunomodulatory actions of adipose-derived mesenchymal stem cells.
Dr. Delgado is the author of 160 publications in the field of neuroimmunology (cited more than 7000 times, personal H-index of 52, cumulative impact factor around 800).
He has licensed three patents for the treatment of inflammatory disorders with neuropeptide- and stem cell-based therapies, which were translated in many clinical trials.
[source: https://www.michaeljfox.org/researcher/mario-delgado-phd]
As a neuroimmunologist, his main research focus has been to understand the bidirectional communication that exists between immune and neuroendocrine systems. A primary objective of the Delgado laboratory is to identify endogenous anti-inflammatory factors, mainly neuropeptides and hormones, that are produced under inflammatory and autoimmune conditions, with the aim of identifying therapeutic agents for immune disorders where tolerance is compromised.
Other Links
Professor James Baraniuk
Georgetown University, USAProfessor of Medicine at Georgetown University Medical Centre, washington, USA
James N. Baraniuk was born in Alberta, Canada, south of Banff. He earned his honours degree in chemistry and microbiology, medical degree, and
unique bachelor's degree in medicine (cardiology) at the University of Manitoba, Winnipeg, Canada.
Thereafter, he moved to Akron, OH, USA, for his internship and internal medicine residency at St Thomas Hospital.
After another year of internal medicine residency at Duke University Medical Center, Durham, NC, he trained with Dr C.E. Buckley, III, in allergy and clinical immunology. He moved to the laboratory of Dr Michael Kaliner at the National Institute of Allergy and Infectious Diseases, Bethesda, MD, and there began his long-standing collaboration with Dr Kimihiro Ohkubo.
After 2 years studying neuropeptides, he joined Dr Peter Barnes' laboratory at the National Heart and Lung Institute, Brompton Hospital, London, UK. Dr Baraniuk returned to Washington, DC, and Georgetown University, where he is currently Associate Professor with Tenure in the Department of Medicine.
-
References
Professor Olav Mella
Haukeland University Hospital, Bergen, NorwayProfessor. Olav Mella
Professor. Olav Mella of Haukeland University Hospital in Bergen, Norway began his investigation of Rituximab’s effects on CFS after treating several Hodgkin’s Lymphoma patients who had long standing cases of CFS prior to developing cancer.
Professor Mella has performed clinical trials to test the benefit of B-cell depletion therapy using Rituximab in ME/CFS patients.
Professor Mella and Dr Fluge have published a paper "Benefit from B-Lymphocyte Depletion Using the Anti-CD20 Antibody Rituximab in Chronic Fatigue Syndrome. A Double-Blind and Placebo-Controlled Study"
Dr Øystein Fluge
Haukeland University Hospital, Bergen, NorwayØystein Fluge received medical degree in 1988 at the University of Bergen, and is a specialist in oncology since 2004.
He has worked as a Research Fellow with support from the Norwegian Cancer Society and is now chief physician at the Cancer Department, Haukeland University Hospital.
Doctoral work emanates from the Surgical Institute and Department of Molecular Biology, University of Bergen.
.-
References
Dr Daniel Peterson
Simmaron Research, USADr Daniel L. Peterson, Whittemore Peterson Institute for Neuroimmune Diseases, Reno, Nevada, USA
Daniel L. Peterson, M.D., is an internist in Incline Village, Nevada and recognized medical expert on CFS/ME. Dr. Peterson is founder of Simmaron Research, and serves on its Scientific Advisory Board. Dr. Peterson has devoted 25 years of his clinical career to diagnosing and caring for patients with CFS/ME and related neuroimmune disorders, and collaborating with researchers to better understand the illness. Dr. Peterson’s repository of more than 1,000 patient biological samples and records is a rich resource for research studies. His experience as both a clinician and a research collaborator provides a unique perspective on CFS/ME for developing translational science.
With over 25 years of medical practice, Dr Daniel L. Peterson has become a sought-after internist for diagnosing difficult and complex medical cases.
When several patients in Incline Village became ill with symptoms that resembled persistent mononucleosis, Daniel Peterson was one of the first physicians to recognize an outbreak of what is known as ME/Chronic Fatigue Syndrome (ME/CFS). He became a pioneering physician and researcher in understanding the biological characteristics and methods for diagnosing, managing and treating ME/CFS. He has also performed major studies of Ampligen as a treatment for ME/CFS, and studying the possible role of human herpes virus 6 (HHV-6) in CFS patients.
Dr. Peterson's experience as both a clinician and a research collaborator provides a unique perspective on CFS/ME for developing translational science.
Other Links
Professor Indre Bileviciute Ljungar
Karolinska Institute, SwedenProfessor Indre Bileviciute Ljungar
Professor Karolinska Institute Department of Clinical Sciences, Danderyd Hospital, Stockholm, Sweden
Dr Andreas Kogelnik
Open MedicineDr Andreas Kogelnik
Dr Andreas Kogelnik is the Founding Director of the Open Medicine Institute, a collaborative, community-based translational research institute dedicated to personalized medicine with a human touch while using the latest advances in medicine, informatics, genomics, and biotechnology.
The Institute works closely with the Open Medicine Clinic and other clinics to conduct research and apply new knowledge back into clinical practice.
Dr. Kogelnik received his M.D. from Emory University School of Medicine in Atlanta and his Ph.D. in bioengineering/bioinformatics from the Georgia Institute of Technology. Subsequently, he completed is residency in Internal Medicine and a Fellowship in Infectious Diseases at Stanford University and its affiliated hospitals. Following his clinical training, he remained at Stanford with NIH funding to engage in post-doctoral research in microbiology, immunology and bioinformatics with Dr. Ellen Jo Baron and Dr. Stanley Falkow, where he explored host-response profiles in severely ill patients.
Together with Dr. José Montoya, he was instrumental in the conception, design, and execution of the EVOLVE study - a placebo-controlled, double-blind study of a subset of chronic fatigue syndrome patients with evidence of viral infection.
Dr. Kogelnik worked with Dr. Atul Butte in translational informatics to determine patterns that indicated a high risk for adverse events in paediatric patients at Lucille Packard Children's Hospital.
H
e is the Medical Director of the Open Medicine Clinic - a community-based research clinic focussed on chronic infectious diseases, neuroimmune disease, and immunology. Dr. Kogelnik has published numerous scientific papers and book chapters, is an Editor of Computers in Medicine and Biology, and is a Consulting Assistant Professor at Stanford University. With the Open Medicine Institute, he has led the formation of CFS and Lyme Registries and Biobanks as well as creating an infrastructure for providers to collect better data and implement clinical trials across a network of sites.
Plenary Session
At the end of the conference more questions were taken from the audience with Dr Ian Gibson mediating. .
We hope that you have enjoyed the conference videos.
Invest in ME Research is a charity of volunteers attempting to make progress in research into ME

We welcome support to enable us to continue our efforts.
-
IiMER Links















